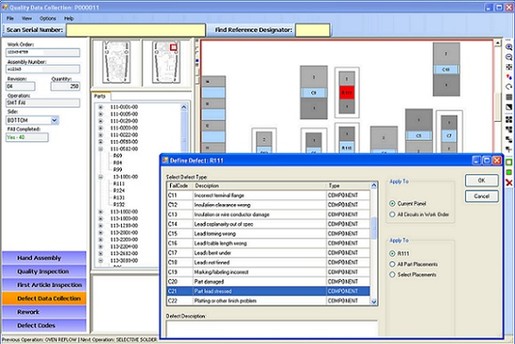
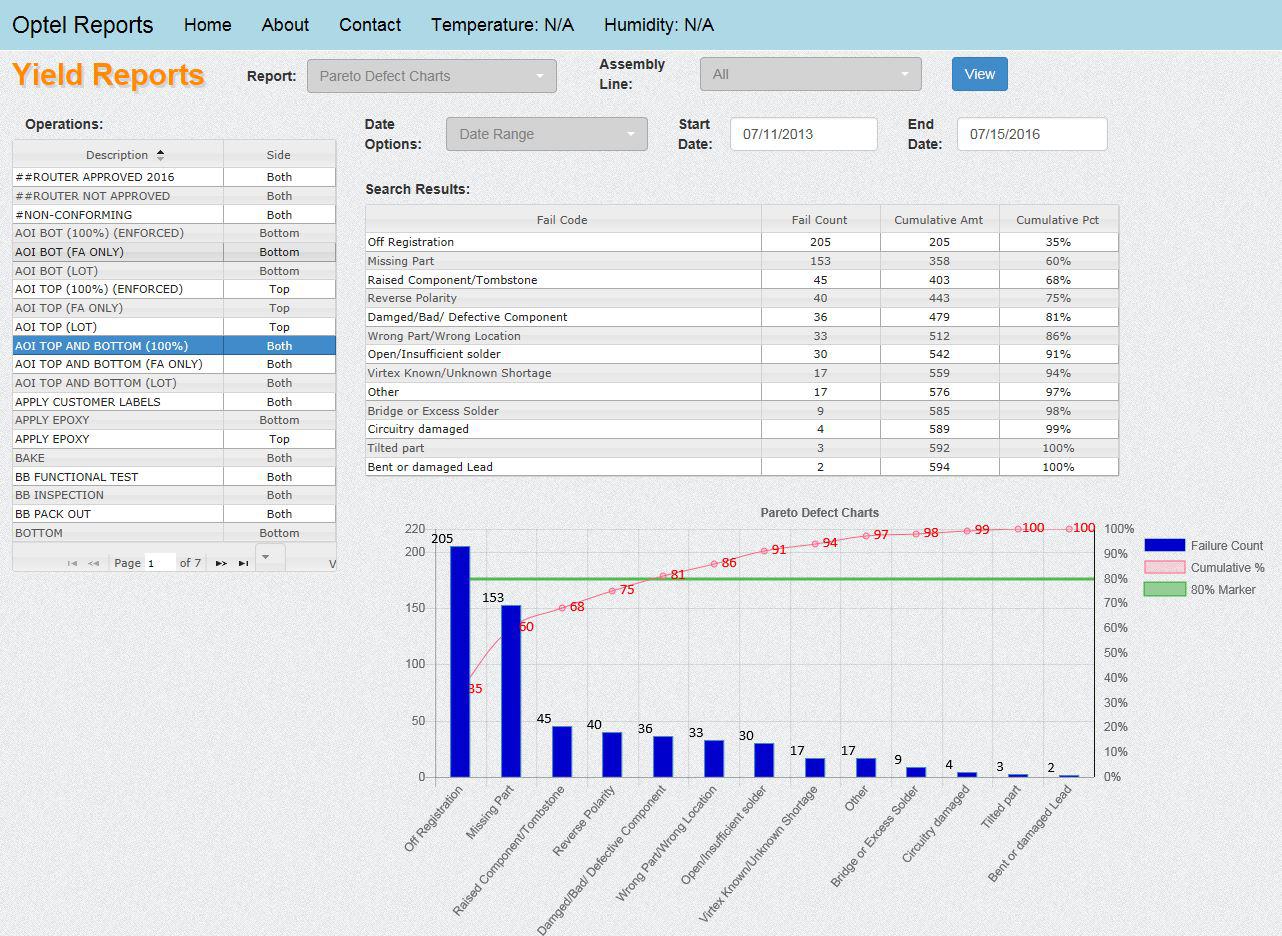
Optel’s Quality Management Module permits automated and manual collection of defect information, defect code management, yield calculations, first article inspection data, and alarms. A defect automatically collected from a test machine is further defined and its correction described using this module. A graphical board layout is used to support defect data collection and first article inspection. A textual and graphical description of the defect is saved into Optel database.